Dassault Systèmes Living Heart Project reaches next milestones in improving patient care
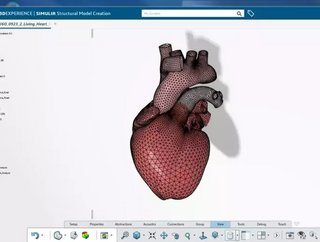
With ambitions to drive the creation and use of simulated 3D personalised hearts in the treatment, diagnosis and prevention of heart diseases, Dassault Systèmes has defined the milestones within its Living Heart Project.
Available through Dassault Systèmes 3DEXPERIENCE cloud platform, the project is targeted at the scientific and medical community, who seek to find faster, targeted ways to improve patient care through new partnerships. The platform offers the speed and flexibility of high-performance computing (HPC) to even the smallest medical device companies.
The Living Heart Project has grown to more than 95-member organisations worldwide including medical researchers, practitioners, device manufacturers and regulatory agencies united in a mission of open innovation to solve healthcare challenges.
The project has supported 15 research grant proposals by providing access to the model, associated technologies and project expertise. Novel use of the model to understand heart disease and study the safety and effectiveness of medical devices has appeared in eight articles published in peer-reviewed journals to date.
Related stories
- Anthem Inc’s new IT hub will support its growing consumer healthcare focus
- The use of AI will transform patient outcomes, report finds
- MyMedicNow launches mobile healthcare app to bridge gap between patients and doctors
Through the platform, any life sciences company can access a complete, on-demand HPC environment to scale up virtual testing securely and collaboratively while managing infrastructure costs. This also crosses an important boundary toward the use of the Living Heart directly in a clinical setting.
“Medical devices need thousands of tests in the development stage,” said Joe Formicola, President and Chief Engineer, Caelynx. “With the move of the Living Heart to the cloud, effectively an unlimited number of tests of a new design can be carried out simultaneously using the simulated heart rather than one at a time, dramatically lowering the barrier to innovation, not to mention the time and cost.”
Since signing a 5-year agreement with the FDA in 2014, Dassault Systèmes will continue to align with the regulatory agency on the use of simulation and modelling to accelerate approvals. “Modelling and simulation plays a critical role in organizing diverse data sets and exploring alternate study designs. This enables safe and effective new therapeutics to advance more efficiently through the different stages of clinical trials,” Food & Drug Administration Commissioner Dr. Scott Gottlieb has said.






